How the Detector Operates
Luminescence Detection
Luminescence, the emission of light, occurs when molecules change from an excited state to their ground state. Molecules can be excited by different forms of energy, each with its own excitation process. For example, when the excitation energy is light, the process is called photoluminescence.
In basic cases, the emission of light is the reverse of absorption, see Absorption of Light Versus Emission of Light . With sodium vapor, for example, the absorption and emission spectra are a single line at the same wavelength. The absorption and emission spectra of organic molecules in solution produce bands instead of lines.
When a more complex molecule transforms from its ground energy state into an excited state, the absorbed energy is distributed into various vibrational and rotational sub-levels. When this same molecule returns to the ground state, this vibrational and rotational energy is first lost by relaxation without any radiation. Then the molecule transforms from this energy level to one of the vibrational and rotational sub-levels of its ground state, emitting light, see Relationship of Excitation and Emission Wavelengths. The characteristic maxima of absorption for a substance is its λEX, and for emission its λEM.
Photoluminescence is the collective name for two phenomena, fluorescence and phosphorescence, which differ from each other in one characteristic way — the delay of emission after excitation. If a molecule emits light 10-9 to 10-5 seconds after it was illuminated then the process was fluorescence. If a molecule emits light longer than 10-3 seconds after illumination then the process was phosphorescence.
Phosphorescence is a longer process because one of the electrons involved in the excitation changes its spin, during a collision with a molecule of solvent, for example. The excited molecule is now in a so-called triplet state, T, see Phosphorescence Energy Transitions.
The molecule must change its spin back again before it can return to its ground state. Since the chance of colliding with another molecule with the necessary spin for change is slight, the molecule remains in its triplet state for some time. During the second spin change the molecule loses more energy by relaxing without radiation. The light which is emitted during phosphorescence therefore has less energy and is at a longer wavelength than fluorescence.
Formula:
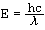
where
E | Energy |
h | Planck's constant |
λ | Wavelength |
c | speed of light |
base-id: 3586867211
id: 3586867211