How to Achieve Higher Resolution
Increased resolution in a separation will improve the qualitative and quantitative data analysis, allow more peaks to be separated or offer further scope for speeding up the separation. This section explains how resolution can be increased by examining the following points:
Optimize selectivity
Smaller particle-size packing
Longer columns
Shallower gradients, faster flow
Resolution between two peaks is described by the resolution equation:
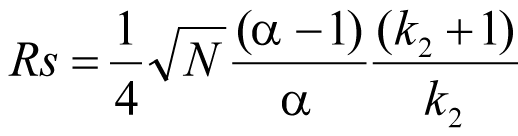
where
Rs=resolution,
N=plate count (measure of column efficiency),
α=selectivity (between two peaks),
k₂=retention factor of second peak (formerly called capacity factor).
The term that has the most significant effect on resolution is the selectivity, α. In practice, varying this term involves changing the type of stationary phase (C18, C8, phenyl, nitrile etc.), the mobile phase, and temperature to maximize the selectivity differences between the solutes to be separated. This is a substantial piece of work that is best done with an automated method development system, which allows assessment of a wide range of conditions on different columns and mobile phases in an ordered scouting protocol. This section considers how to get higher resolution with any chosen stationary and mobile phases. If an automated method development system was used in the decision on phases, it is likely that short columns were used for fast analysis in each step of the scouting.
The resolution equation shows that the next most significant term is the plate count or efficiency, N, which can be optimized in several ways. N is inversely proportional to the particle size and directly proportional to the length of a column. Smaller particle size and a longer column thus result in a higher plate number. The pressure rises with the inverse square of the particle size and proportionally with the length of the column. This is the reason that the 1290 LC System was designed to go to 1300 bar so that it can run sub-2-micron particles and column length can be increased to 100 mm or 150 mm. There are even examples of 100 mm and 150 mm columns linked to give 250 mm length. Resolution increases with the square root of N so doubling the length of the column will increase resolution by a factor of 1.4 . What is achievable depends on the viscosity of the mobile phase as this relates directly to the pressure. Methanol mixtures will generate more backpressure than acetonitrile mixtures. Acetonitrile is often preferred because peak shapes are better and narrower in addition to the lower viscosity but methanol generally yields better selectivity (certainly for small molecules less than about 500 Da). The viscosity can be reduced by increasing the temperature but it should be remembered that this can change the selectivity of the separation. Experiment will show if this leads to increase or decrease in selectivity. As flow and pressure are increased, it should be remembered that frictional heating inside the column will increase and that can lead to slightly increased dispersion and possibly a small selectivity change both of which could be seen as a reduction in resolution. The latter case might be offset by reducing the temperature of the thermostat by a few degrees and again experiment will reveal the answer.
The van Deemter curve shows that the optimum flow rate through an STM column is higher than for larger particles and is fairly flat as the flow rate increases. Typical, close to optimum, flow rates for STM columns are: 2 ml/min for 4.6 mm i.d.; and 0.4 ml/min for 2.1 mm i.d. columns.
In isocratic separations, increasing the retention factor, k, results in better resolution because the solute is retained longer. In gradient separations the retention is described by k* in the following equation:
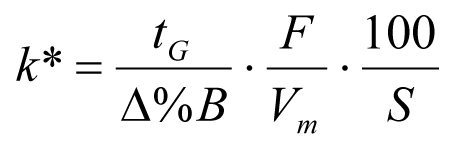
where:
k* = mean k value,
tG = time length of gradient (or segment of gradient) (min),
F = flow (mL/min),
Vm = column delay volume,
Δ%B = change in fraction of solvent B during the gradient,
S = constant (ca. 4 – 5 for small molecules).
This shows that k and hence resolution can be increased by having a shallower gradient (2 to 5 %/min change is a guideline), a higher flow rate, and a smaller column volume. This equation also shows how to speed up an existing gradient – if the flow is doubled but the gradient time is halved, k* remains constant, and the separation looks the same but happens in half the time. Recently published research has shown how a shorter STM column (at temperatures above 40 °C) can generate higher peak capacity than a longer STM column by virtue of running it faster. (See Petersson et al., J.Sep.Sci, 31, 2346-2357, 2008, Maximizing peak capacity and separation speed in liquid chromatography)
base-id: 3803380875
id: 18014402312862859-2